We value your feedback! Take our quick client satisfaction survey here.

~60% of infants admitted to level IV NICUs admissions are likely eligible for rapid genome sequencing,1 yet less than 5% of eligible patients receive rapid testing.2 For these critically ill infants, every moment matters. Rapid genome sequencing can provide an early and precise diagnosis–enabling timely interventions, reducing hospital stays, and improving outcomes.3,4
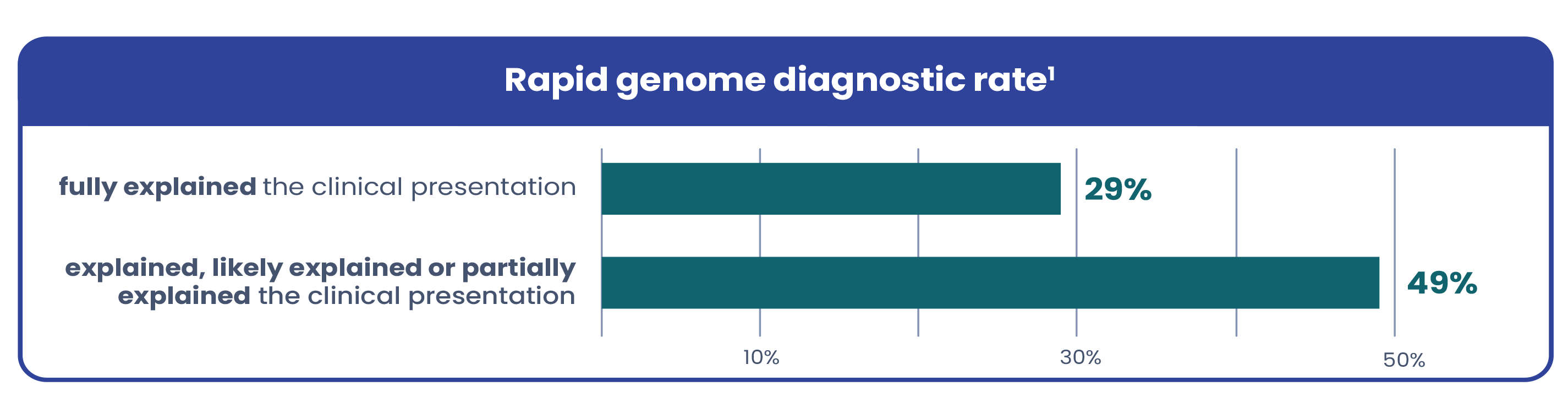
Due to variable and complex presentations, genetic conditions in critically ill infants are often hard to recognize based on symptoms alone. Current testing workflows are too narrow, leaving many patients undiagnosed. A recent study in The American Journal of Human Genetics found that among the infants who received a genetic diagnosis via rapid genome with a broad testing protocol:1
42% would have been missed with conventional workflows
24% were not suspected to have a genetic condition and would not have been offered genetic testing with conventional workflows

Testing all infants whose clinical condition is not fully explained by trauma, infection, or prematurity leads to more diagnoses and maintains a high diagnostic yield.1
Change medical management for up to 87% of babies1
Reduce healthcare costs up to $15,786 per child5

Genetics is a constantly evolving field, with new disease-gene relationships discovered regularly. Genome sequencing results can be reanalyzed to leverage these scientific advancements and improve diagnostic outcomes. GeneDx provides one reanalysis at no additional charge per genome order, recommended at least one year after the original analysis to maximize gene discovery. Reanalysis is also beneficial when there are significant updates to the patient’s phenotype, such as new imaging or biochemical results.
*Turnaround times are estimates and begin once the sample(s) begin processing at the GeneDx lab and could be extended in situations outside GeneDx’s control.
†Case study is based on GeneDx patient testing, with all identifying information removed.
References: 1. Wenger T, Scott A, Kruidenier L, et al. Am J Hum Genet. 2025; 112, 1–15. 2. Kingsmore, Stephen F et al. NPJ genomic medicine vol. 9,1 17. 27 Feb. 2024. 3. Farnaes L, Hildreth A, Sweeney NM, et al. NPJ Genom Med. 2018 Apr 4;3:10. 4. NICUSeq Study Group et al. JAMA Pediatr. 2021;175(12):1218-1226. 5. Dimmock D, Caylor S, Waldman B, et al. Am J Hum Genet. 2021 Jul 1;108(7):1231-1238. 6. Morton SU, Christodoulou J, Costain G, et al. JAMA Neurol. 2022 Apr 1;79(4):405-412. 7. Manickam K, McClain MR, Demmer LA, et al. Genet Med. 2021;23(11):2029-2037. 8. Smith L, Malinowski J, Ceulemans S, et al. J Genet Couns. 2022 Oct 24. 9. Rodan LH, et al. Pediatrics. 2025; e2025072219. doi:10.1542/peds.2025-072219.